Pyrrolo[3,2-b]quinoxaline Derivatives as Types I1/2 and II Eph Tyrosine Kinase Inhibitors: Structure-Based Design, Synthesis, and in Vivo Validation.
Unzue, A., Dong, J., Lafleur, K., Zhao, H., Frugier, E., Caflisch, A., Nevado, C.(2014) J Med Chem 57: 6834
- PubMed: 25076195
- DOI: https://doi.org/10.1021/jm5009242
- Primary Citation of Related Structures:
4P4C, 4P5Q, 4P5Z - PubMed Abstract:
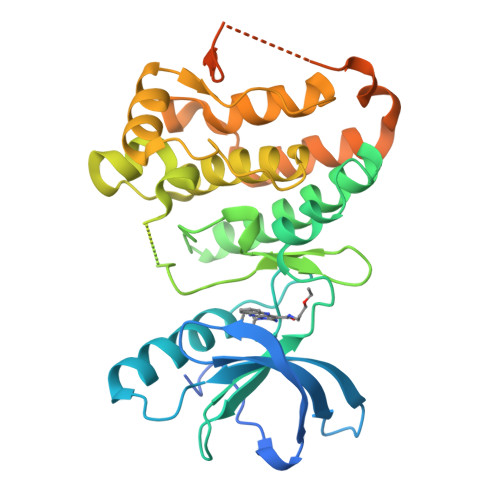
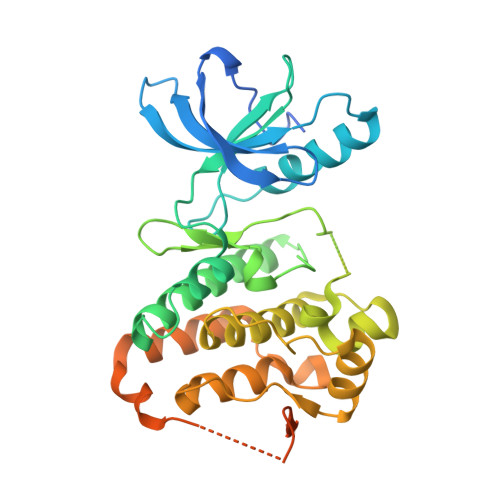
The X-ray crystal structures of the catalytic domain of the EphA3 tyrosine kinase in complex with two type I inhibitors previously discovered in silico (compounds A and B) were used to design type I1/2 and II inhibitors. Chemical synthesis of about 25 derivatives culminated in the discovery of compounds 11d (type I1/2), 7b, and 7g (both of type II), which have low-nanomolar affinity for Eph kinases in vitro and a good selectivity profile on a panel of 453 human kinases (395 nonmutant). Surface plasmon resonance measurements show a very slow unbinding rate (1/115 min) for inhibitor 7m. Slow dissociation is consistent with a type II binding mode in which the hydrophobic moiety (trifluoromethyl-benzene) of the inhibitor is deeply buried in a cavity originating from the displacement of the Phe side chain of the so-called DFG motif as observed in the crystal structure of compound 7m. The inhibitor 11d displayed good in vivo efficacy in a human breast cancer xenograft.
Organizational Affiliation:
Department of Chemistry and ‡Department of Biochemistry, University of Zürich , Winterthurerstrasse 190, CH-8057 Zürich, Switzerland.